Frequently Asked Questions
ACHC Process
ACHC Certification is a focused review and evaluation of a defined program within a healthcare organization as measured against recognized standards for specialty care.
Each provider or supplier organization delivers care/supports the needs of a defined patient population. For some ACHC programs, standards are categorized under Services to ensure relevance to the care provided by organizations seeking accreditation.
Log into your customer portal (details below) to access, complete, and submit your accreditation or certification application. If you have questions or need assistance, call (855) 937-2242 or email [email protected].
Customer Central for the following programs: Ambulatory Care, Assisted Living, Behavioral Health, Dentistry, DMEPOS, Home Care, Home Health, Home Infusion Therapy, Hospice, Palliative Care, Pharmacy, PCAB, Renal Dialysis, and Sleep Accreditation and Telehealth Certification.
Compass for the following programs: Acute Care Hospital, Ambulatory Surgery Center, Clinical Laboratory, Critical Access Hospital, and Office-Based Surgery Accreditation and Joint Replacement, Lithotripsy, Stroke, and Wound Care Certification.
The time frame to complete the accreditation process depends on your organization submitting all required information. Your personal Account Advisor is available to assist with survey schedule needs.
Generally, your organization can expect a survey less than 90 days from the submission date of all required information. For new organizations seeking initial accreditation, an accelerated option may be available. Call (855) 937-2242 or email [email protected] for information about ACHC’s TIME program.
To help ensure that your organization experiences no lapse in accreditation, we recommend that you submit your renewal application six months before your expiration date. This gives your organization time to prepare for your survey and also allows time for ACHC’s scheduling team to identify a great Surveyor/survey team in a reasonable time frame.
The Preliminary Evidence Checklist (PER) lists items that must be completed and submitted before the accreditation process continues. The form is required only for community-based case organizations seeking initial accreditation and does not apply to ACHC acute care accreditation programs.
Items required vary based on the accreditation program and services requested by an applicant organization. A representative of your organization must sign the form, to attest that your organization meets all requirements and is ready to be surveyed. The PER is available to download from your customer portal.
If your organization wants to become or remain a Medicare-approved provider or supplier, your “deemed status” survey will be unannounced. Other surveys, including on-site accreditation or certification surveys and virtual surveys (where available), are scheduled in collaboration with the applicant organization.
For more information, contact your Account Advisor.
On-Site Surveys
Deemed status accreditation surveys: No. The Centers for Medicare & Medicaid Services (CMS) no longer allows accreditation organizations to alert healthcare organizations before an unannounced survey for deemed status accreditation.
ACHC previously notified community-based clinical programs 30 minutes prior to arrival for a deemed status survey.
In accordance with new CMS rules, your ACHC Surveyor will arrive without prior contact within your posted hours of operation to conduct a survey.
The CMS change is intended to ensure that organizations seeking Medicare initial certification or recertification are open and operational during established working hours.
DMEPOS providers are not affected by the CMS change unless services are provided as part of another deemed program, such as Home Infusion Therapy Accreditation, or are provided under a company/system affected by the CMS change. Otherwise, the process is unchanged for DMEPOS providers.
Non-deemed accreditation surveys: Yes, for most organizations. Community-based organizations will continue to be contacted before a survey for non-deemed accreditation — unless your state requires an unannounced survey or your non-deemed survey is conducted at the same time as a deemed accreditation survey for another service.
Your Account Advisor can provide additional information. If applicable, ACHC will notify organizations on the morning of the survey, before the Surveyor arrives. As a safety protocol, we will notify the contact person specified on your application.
Virtual Surveys
The ACHC virtual survey coordinator will contact your organization via email 48 business hours before your survey.
Your ACHC survey is an independent assessment of your organization’s compliance with recognized national standards for quality and safety conducted with an educational approach designed to maximize your organization’s capacity for excellence.
The survey begins with an opening conference at which introductions are made, the survey agenda is finalized, and expectations are established.
Your Surveyor/survey team will assess compliance in three ways:
- Through direct observation of care and services being provided.
- Through interviews of select personnel, employees, and contracted individuals who provide care on behalf of your organization. Also, if applicable, through patient interviews.
- Through review of documentation that supports compliance with ACHC Standards and applicable federal and state regulations. The same materials will be reviewed during an on-site or virtual survey.
The survey ends with a closing conference. Your Surveyor/survey team will review the preliminary summary of findings. Surveyors do not make the accreditation determination. After your survey, the survey results will be submitted to the relevant ACHC office-based team for review and processing.
As your partner, ACHC is always here to help. After applying for accreditation or certification, your personal Account Advisor is dedicated to answering questions and meeting your needs. Clinical experts are also available to assist you. Program-specific information can be found on the Customer Central portal or through your program team.
In addition, a comprehensive library of educational tools and resources to help you prepare for and maintain accreditation is offered by ACHCU, the education division of ACHC. Information on program-specific accreditation tools, workshops, webinars, and other educational materials can be found at achcu.com.
All ACHC Surveyors are subject-matter experts for the programs they survey. They have extensive experience, credentials, and education to provide program-specific expertise. All Surveyors receive extensive on-site orientation and a preceptorship, as well as continuous ongoing training.
ACHC does not charge any annual fees for organizations to remain accredited.
ACHC does not charge for Surveyor expenses. The cost of ACHC accreditation is a single, inclusive fee that creates greater transparency and ease of budgeting for your organization.
Assisted Living
Accreditation is used to measure quality of organizations. Achieving accreditation will help your organization highlight strengths, reaffirm your commitment to compliance, drive continuous performance improvement, and differentiate your organization from competitors.
Accreditation provides residents and their families assurance they are selecting a community that values quality, safety, and exceptional care. This offers a level of confidence in their choice in a value-based community that demonstrates the willingness to invest in operational excellence that exceeds minimal expectations.
Most states do require a facility license that includes bed capacity and any special services offered, such as a Memory Care Unit. A state license that reaches its expiration date requires reapplication for accreditation.
Most state funding for low-income/eligible recipients, such as Medicaid, covers a portion of assisted living services related to personal care assistance.
The length of the survey depends on the facility’s current census. At a minimum, a survey will take two (2) days for a census of 80 or fewer residents, including one (1) day for the Life Safety Code (LSC) Survey of environmental and physical facilities and both days for the Nurse Survey. A survey will take three (3) days for a census of 81 or more residents.
Surveys for Assisted Living Accreditation are unannounced.
The survey team typically consists of two Surveyors — a healthcare Surveyor and a Life Safety Code Inspector/Surveyor. However, ACHC reserves the right to include additional Surveyors in training/preceptee as part of the survey team.
The Memory Care Unit licensed for the same facility is included with the standard survey and does not require a separate survey.
ACHC and ACHCU provide resources to assist you with preparing for your survey. ACHCU, the educational division of ACHC, offers educational materials and training resources. ACHC also offers Certified Consultant Training, and we list Consultants on our website, should you wish to seek their services independently.
To be eligible to apply for this distinction, an organization must collect data on resident outcomes for the most recent four consecutive quarters and have it analyzed by an ACHC-approved vendor. ACHC Assisted Living Accreditation is also required.
Ambulatory Surgery Center
The duration of an ACHC Ambulatory Surgery Center (ASC) Accreditation survey is typically for two days with two ACHC Surveyors. On rare occasions, a solo Surveyor may be sent out for three or four days if one of the other Surveyors cannot participate due to an emergency. The exact number of days will be determined by ACHC based on an assessment of the ASC’s scope of services and other factors.
An ambulatory surgery center (ASC) seeking Medicare deemed status should expect an unannounced survey, per CMS regulations. For an ASC that simply wants to achieve accreditation without Medicare deemed status, ACHC typically conducts announced surveys, meaning the date of the survey is scheduled in advance with the facility.
Yes. ACHC provides resources and tools to help organizations prepare for accreditation surveys. This includes a free self-assessment tool that allows new applicants to conduct their own mock surveys and set themselves up for success. In addition to tools, an ambulatory surgery center will have access to our Clinical Review Specialists team that works with you leading up your survey to ensure that your survey is a success.
ACHC requires that the ambulatory surgery center (ASC) serve at least 10 patients before the survey. Additionally, ACHC requires that you have one or two active procedures on the day(s) of the survey. Per CMS, this allows ACHC to review an appropriate number of closed records (10) as well as an open record.
Behavioral Health
Accreditation is used to measure the quality of organizations. Achieving accreditation will help your organization highlight strengths, reaffirm your commitment to compliance, drive continuous performance improvement, and differentiate your organization from competitors.
Some states, such as Maryland, require accreditation in order for an organization to apply for or receive a license.
An organization must have provided care to a minimum of three (3) service recipients and have one (1) active for a single service, or five (5) service recipients served with three (3) active for multiple services. This applies to organizations currently licensed and providing services.
The number of survey days depends on the type of survey, the number of services to be accredited, and the number of locations/branches. The number of survey days can range from one (1) to five (5) or more.
Currently, ACHC Behavioral Health Accreditation Surveys are announced.
The number of Surveyors assigned depends on number of locations. Typically, one (1) Surveyor is present. However, on a rare occasion if a customer has a large number of locations, ACHC may assign two (2) Surveyors to facilitate multiple locations. Also, ACHC reserves the right to assign a new Surveyor in training/preceptee to be present for an organization’s survey.
Because there may be different survey requirements for each state, organizations with multiple locations should contact ACHC to determine the type and number of survey(s) required.
ACHC and ACHCU provide resources to assist you with preparing for your survey. ACHCU, the educational division of ACHC, has educational materials, webinars, and training resources available. ACHC also offers Certified Consultant Training and lists Consultants on our website should you seek their services independently.
Clinical Laboratory
CLIA certification is required for facilities that test clinical specimens for the purpose of diagnosis, treatment, or prevention of disease. If your facility only collects specimens to be sent out for testing at another facility, then CLIA certification is not required for your facility. Laboratories performing research-only testing, laboratories operated directly by the federal government (e.g., Veterans Affairs, Department of Defense), or laboratories testing for the hiring or termination of employees are CLIA-exempt and do not require CLIA certification.
There are four certification types, depending on the type of testing performed:
- Certificate of Waiver (COW): Issued to a laboratory that performs only CLIA-waived tests.
- Certificate for Provider-Performed Microscopy (PPM) Procedures: Allows the facility to perform only CLIA-waived and specific microscopy PPM tests. Certificate of Registration is issued to allow the laboratory to perform CLIA-waived, PPM, and non-waived (moderate- or high-complexity) testing until the laboratory is surveyed (inspected). Once the laboratory is inspected and found in compliance, a final certificate of Compliance or Accreditation is issued.
- Certificate of Compliance (COC): Issued after the laboratory is inspected and found in compliance. This type of certificate allows the laboratory to perform CLIA-waived, PPM, and non-waived (moderate- and/or high-complexity) testing. Inspection is conducted by the CLIA Program.
- Certificate of Accreditation (COA): Issued once the laboratory is inspected and found in compliance. This type of certificate allows the laboratory to perform CLIA-waived, PPM, and non-waived (moderate- and/or high-complexity) testing. Inspection is conducted by a laboratory-selected, CMS-approved accrediting agency such as ACHC.
DMEPOS
Agencies can provide five (5) mock files at the time of survey if equipment or supplies have not been provided. A mock file is a sample patient file that should be set up to include all required information/content that would be present in a true patient file.
You can apply to the Centers for Medicare & Medicaid Services (CMS) for your DMEPOS Medicare number after you have been approved for accreditation.
Visit the CMS website for additional information on the DMEPOS supplier Medicare enrollment process. You also can download a copy of CMS-855S from the CMS website.
The DMEPOS Quality Standards are standards created by the Centers for Medicare and Medicaid Services (CMS) that providers of durable medical equipment, prosthetics, orthotics, and supplies must comply with to attain accreditation. Accrediting organizations, such as Accreditation Commission for Health Care (ACHC), must have standards that address all the Quality Standards.
Each state has different licensure, registration, and/or certification requirements for the provision of DMEPOS products, the handling of oxygen, the sterilization of upholstered items, and the points when licensed healthcare professionals must be used. Consult each state licensure board and the DMEPOS Licensure Database to get information on required licenses, certificates, etc.
Each state determines if out-of-state providers are required to obtain licensure or maintain an in-state location to ship DMEPOS products into the state. Consult each state licensure board and the DMEPOS Licensure Database to get information on required licenses, certificates, etc.
CMS requires most DMEPOS suppliers to obtain a surety bond before applying for accreditation and Medicare billing enrollment. The bond requirement aims to reduce billing fraud and abuse. DMEPOS suppliers must maintain a $50,000 surety bond for each practice location with a National Provider Identifier (NPI) number. The bonds provide CMS an avenue to recover funds if suppliers are found to be noncompliant with Medicare billing requirements. The bond requirement also is included in the DMEPOS Supplier Standards. For final determination of security bond requirements or assistance with surety bonds, consult the national provider enrollment (NPE) contractor for your area: NPE East or NPE West. CMS also provides a PDF of surety bond FAQs you can download.
CMS requires each DMEPOS supplier to maintain comprehensive liability insurance of at least $300,000. The insurance must cover the supplier’s place of business, employees, and customers. If the supplier manufacturers its own products, the insurance must cover product liability and completed operations as well. This requirement is contained in the DMEPOS Supplier Standards.
ACHC and DMEPOS Quality Standards require after-hours coverage based on the products/services being provided. If respiratory products are being provided, 24/7 coverage is required. Many states have their own requirements for after-hours coverage. State regulations should be reviewed.
DMEPOS Supplier Standards require all suppliers to maintain a primary business telephone listed under the name of the business in a local directory or a toll-free number available through directory assistance. The exclusive use of a beeper, answering machine, answering service, or cellphone during posted business hours is prohibited. Business lines can be transferred to a cellphone for after-hours coverage.
DMEPOS Supplier Standards require each supplier to fill orders from their own inventory or to contract with other companies to do so. If inventory is not on site, you will need to show a contract, line of credit, or other agreement with a vendor to verify that the product can be ordered/obtained in time to fulfill the order.
The requirement for an RT or a nurse to perform setups, mask fits, etc., is based on state regulations. Consult the regulatory board for the practice of respiratory care in your state.
Anyone who maintains or tests equipment for proper function, performs minor repairs, or sets up or educates patients on the use of DMEPOS items will need documented training and competency to do so. For warranty or manufacturer-approved repairs, proof of training by the manufacturer will be needed by at least one technician. Others may have documentation of training and competency to do so unless not permitted by the manufacturer. For Complex Rehabilitation & Assistive Technology (RTS) suppliers, DMEPOS Quality Standards dictate that repair technicians are trained by manufacturers and complete at least 10 hours annually of continuing education specific to rehabilitation technology.
There are many ways to obtain feedback. Feedback can be obtained in the form of surveys, phone calls, communications during evaluations or visits, hotlines, or a variety of other means.
HME
Home Medical Equipment (HME) accreditation covers what many refer to as a full-line HME provider or those that specialize in the provision of a specific product category such as respiratory equipment. HME accreditation includes the largest number of product codes
MSP
Medical Supply Provider (MSP) accreditation routinely refers to supplies that are sold and shipped to the patient and includes only the rental of nebulizers. In a few cases, it does include rental equipment such as enteral/infusion pumps, if these items are maintained by the manufacturer. Continuous positive airway pressure (CPAP) machines are included because organizations provide the associated disposable products that go with the equipment, in which case the organization should indicate that it is only providing the supplies. MSP accreditation also includes a large number of product codes, with the exception of most rental equipment.
CRTL
Community Retail (CRTL) accreditation is designed specifically for retail pharmacies that provide a small selection of DMEPOS items. Items are mostly sale items, with the exception of nebulizers, which are still rented by Medicare. CRTL accreditation includes a limited number of product codes.
HME Codes
| Code | Description |
| DM01 | Automatic External Defibrillators (AEDs) & Supplies |
| DM02 | Commodes/Urinal/Bedpans |
| DM03 | CPM Devices |
| DM04 | Contracture Treatment Devices: Dynamic Splint |
| DM05 | Blood Glucose Monitors & Supplies – (non-mail order) |
| DM06 | Blood Glucose Monitors & Supplies – (mail order) |
| DM07 | Gastric Suction Pumps |
| DM08 | Heat & Cold Applications |
| DM09 | Hospital Beds – Electric |
| DM10 | Hospital Beds – Manual |
| DM11 | Infrared Heating Pad Systems & Supplies |
| DM12 | External Infusion Pumps |
| DM13 | Insulin Infusion Pumps |
| DM14 | Implanted Infusion Pumps & Supplies |
| DM15 | Negative Pressure Wound Therapy Pumps & Supplies |
| DM16 | Neuromuscular Electrical Stimulators & Supplies |
| DM17 | Osteogenesis Stimulators |
| DM18 | Pneumatic Compression Devices & Supplies |
| DM19 | Speech Generating Devices |
| DM20 | Support Surfaces: Pressure Reducing Beds/Mattresses/Overlays/Pads – new |
| DM21 | Traction Equipment |
| DM22 | TENS & Supplies |
| DM23 | Ultraviolet Light Devices & Supplies |
| DM24 | External Infusion Pump Supplies |
| DM25 | Insulin Infusion Pump Supplies |
| DM26 | Pressure Reducing Beds/Mattresses/Overlays/Pads – Used |
| M01 | Canes & Crutches |
| M02 | Patient Lifts |
| M03 | Power Operated Vehicles (Scooters) |
| M04 | Seat Lift Mechanisms |
| M05 | Walkers |
| M06 | Wheelchairs – Standard Manual |
| M06A | Wheelchairs – Standard Manual Related Accessories & Repairs |
| M07 | Wheelchairs – Standard Power Related Accessories & Repairs |
| M07A | Wheelchairs – Standard Power |
| M10 | Wheelchair Seating Cushions |
| OR03 | Orthoses: Off the Shelf |
| OR04 | Penile Pumps |
| PD03 | Facial Prostheses |
| PD04 | Neurostimulators & Supplies |
| PD06 | Ostomy Supplies |
| PD07 | Somatic Prostheses |
| PD08 | Tracheostomey Supplies |
| PD09 | Urological Supplies |
| PD10 | Voice Prosthetics |
| PD11 | Prosthetic Lenses: Conventional Eyeglasses |
| PD12 | Prosthetic Lenses: Conventional Contact Lenses |
| PD13 | Prosthetic Lenses: Prosthetic Cataract Lenses |
| PE03 | Enteral Nutrients |
| PE04 | Enteral Equipment &/or Supplies |
| PE06 | Parenteral Equipment &/or Supplies |
| R01 | CPAP Devices & Supplies |
| R02 | High Frequency Chest Wall Oscillators & Supplies |
| R04 | IPPB Machines |
| R05 | Intrapulmonary Percussive Ventilation Devices |
| R06 | Mechanical In-Exsufflation Devices |
| R07 | Nebulizer Equipment & Supplies |
| R08 | Oxygen Equipment & Supplies |
| R08a | Oxygen |
| R09 | Respiratory Assist Devices |
| R10 | Respiratory Suction Pumps |
| R12 | Ventilators: All Types – Not CPAP or RAD |
| S01 | Surgical Dressings |
MSP Codes
| Code | Description |
| DM01 | Automatic External Defibrillators (AEDs) & Supplies |
| DM02 | Commodes/Urinal/Bedpans |
| DM04 | Contracture Treatment Devices: Dynamic Splint |
| DM05 | Blood Glucose Monitors & Supplies – (non-mail order) |
| DM06 | Blood Glucose Monitors & Supplies – (mail order) |
| DM08 | Heat & Cold Applications |
| DM11 | Infrared Heating Pad Systems & Supplies |
| DM12 | External Infusion Pumps |
| DM13 | Insulin Infusion Pumps |
| DM14 | Implanted Infusion Pumps & Supplies |
| DM16 | Neuromuscular Electrical Stimulators & Supplies |
| DM17 | Osteogenesis Stimulators |
| DM19 | Speech Generating Devices |
| DM20 | Support Surfaces: Pressure Reducing Beds/Mattresses/Overlays/Pads – new |
| DM21 | Traction Equipment |
| DM22 | TENS & Supplies |
| DM23 | Ultraviolet Light Devices & Supplies |
| DM24 | External Infusion Pump Supplies |
| DM25 | Insulin Infusion Pump Supplies |
| DM26 | Pressure Reducing Beds/Mattresses/Overlays/Pads – Used |
| M01 | Canes & Crutches |
| M04 | Seat Lift Mechanisms |
| M05 | Walkers |
| M06 | Wheelchairs – Standard Manual |
| M06A | Wheelchairs – Standard Manual Related Accessories & Repairs |
| M10 | Wheelchair Seating Cushions |
| OR03 | Orthoses: Off the Shelf |
| OR04 | Penile Pumps |
| PD02 | Cochlear Implants |
| PD03 | Facial Prostheses |
| PD04 | Neurostimulators & Supplies |
| PD06 | Ostomy Supplies |
| PD07 | Somatic Prostheses |
| PD08 | Tracheostomey Supplies |
| PD09 | Urological Supplies |
| PD10 | Voice Prosthetics |
| PD11 | Prosthetic Lenses: Conventional Eyeglasses |
| PD12 | Prosthetic Lenses: Conventional Contact Lenses |
| PD13 | Prosthetic Lenses: Prosthetic Cataract Lenses |
| PE03 | Enteral Nutrients |
| PE04 | Enteral Equipment &/or Supplies |
| PE06 | Parenteral Equipment &/or Supplies |
| R01 | CPAP Devices & Supplies |
| R07 | Nebulizer Equipment & Supplies |
| S01 | Surgical Dressings |
CRTL Codes
| Code | Description |
| DM02 | Commodes/Urinal/Bedpans |
| DM05 | Blood Glucose Monitors & Supplies – (non-mail order) |
| DM06 | Blood Glucose Monitors & Supplies – (mail order) |
| DM08 | Heat & Cold Applications |
| DM13 | Insulin Infusion Pumps |
| DM22 | TENS & Supplies |
| DM25 | Insulin Infusion Pump Supplies |
| M01 | Canes & Crutches |
| M04 | Seat Lift Mechanisms |
| M05 | Walkers |
| M06 | Wheelchairs – Standard Manual |
| M06A | Wheelchairs – Standard Manual Related Accessories & Repairs |
| M10 | Wheelchair Seating Cushions |
| OR03 | Orthoses: Off the Shelf |
| OR04 | Penile Pumps |
| PE03 | Enteral Nutrients |
| PD06 | Ostomy Supplies |
| PD08 | Tracheostomey Supplies |
| PD09 | Urological Supplies |
| R07 | Nebulizer Equipment & Supplies |
| S01 | Surgical Dressings |
| PD13 | Prosthetic Lenses: Prosthetic Cataract Lenses |
| Additional for CRDS | |
| PD01 | Breast Prostheses & Accessories |
| S02 | Diabetic Shoes/Inserts |
| S03 | Diabetic Shoes/Inserts – custom |
Home Care
The organization must have provided care to a minimum of five (5) clients/patients, having three (3) active, or receiving care, at time of survey unless state law requires more.
Yes, you must have provided care for a skilled nursing client/patient for initial accreditation in Home Care Nursing. The client/patient does not need to be active, or receiving care, at the time of survey.
A skilled nursing patient is a patient who requires the skill of a Registered Nurse (RN) to provide the care that is ordered, such as wound care, IV administration, or education.
The aide care plan must be developed by a professional based on state and federal regulations and payor guidelines.
Aides are supervised based on state and federal regulations or payor guidelines, or, at a minimum, every 90 days. The frequency of aide supervision is defined in the home care agency’s policies and procedures.
A Registered Nurse must provide supervision to aides who provide Medicaid Personal Care Services. Other qualified professionals, in accordance with state law, may provide this supervision in other programs.
“Qualified professional” refers to a licensed Registered Nurse, licensed Physical Therapist, licensed Speech Therapist, Certified Occupational Therapist, or person with a bachelor’s or master’s degree in social work, home economics, or closely related helping profession.
Home Health
A home health agency seeking Initial Medicare Certification must have an approved CMS-855A Medicare Enrollment Application – Institutional Providers Form from the Centers for Medicare & Medicaid Services (CMS) to begin the accreditation process.
How many patients do I need to be eligible for an Initial Medicare Certification Home Health Survey?
The organization must have provided care to a minimum of ten (10) patients requiring skilled care (not required to be Medicare beneficiaries). At least seven (7) of the required ten (10) patients should be receiving skilled care from the home health agency at the time of the Medicare survey, unless the care is provided in a medically underserved or rural area as determined by the Regional Office.
You must be providing or have provided skilled nursing care and at least one other therapeutic service such as physical therapy, occupational therapy, speech therapy, home health aide services, and/or medical social services.
To determine if a CON is required for the state in which you want to operate a home health agency, visit the National Conference of State Legislatures CON-Certificate of Need State Laws page online.
The ACHC compliance date is the date on which you acknowledge that your organization was/is/will be in compliance with the ACHC Accreditation Standards. The compliance date does not apply to state and Medicare Conditions of Participation (CoPs) because agencies must always be in compliance with these requirements from the initiation of patient care.
Home Infusion Therapy
The 21st Century Cures Act, which was signed into law on December 13, 2016, called for the creation of a bundled payment for providers that supply home infusion therapy services to Medicare beneficiaries. A requirement of the act stipulated that home infusion suppliers be accredited, beginning January 1, 2021, by an accrediting organization approved by the Centers for Medicare & Medicaid Services (CMS) in order to receive Medicare Part B reimbursement.
Yes, the Centers for Medicare & Medicaid Services (CMS) has recognized ACHC as a national accrediting organization for home infusion therapy suppliers. The decision gives ACHC deeming authority to conduct surveys that meet or exceed Medicare requirements, including reimbursement rules mandated by the 21st Century Cures Act.
Each location is required to have its own National Provider Identifier (NPI) and Medicare Provider Transaction Access Number (PTAN). Accreditation is required for each location.
No. HITS and IRN services both involve the administration of medications via various accesses and ports, with care provided by qualified professional nurses, as allowed by state regulations.
Pharmacy Infusion Nursing Accreditation allows an organization to bill third-party payors. Home Infusion Therapy Accreditation allows an organization to bill Medicare Part B and third-party payors with a physician’s order.
Yes. The Centers for Medicare & Medicaid Services (CMS) requires that all locations receive an on-site survey.
New home infusion therapy suppliers must administer intravenous medications in the home, under the supervision of a Registered Nurse, to at least three clients/patients.
In rural or medically underserved areas, a new home infusion therapy supplier seeking accreditation must provide services to two clients/patients.
Yes. Program-specific educational materials are available on the customer portal. Plus, your personal Account Advisor is dedicated to answering questions and meeting your needs. Clinical experts are also available to assist you.
In addition, a comprehensive library of educational tools and resources to help you prepare for and maintain accreditation is offered by ACHCU, the education division of ACHC. Information on program-specific accreditation tools, workshops, webinars, and other educational materials can be found at achcu.com.
The supplier must be able to meet the provisions defined in the Social Security Act. The Social Security Act (section 1861(iii)(3)(D)(i))defines a supplier as:
- A pharmacy, physician, or other provider of services or supplier licensed by the State in which the pharmacy, physician, or provider of services or supplier furnishes items or services and that:
- Furnishes infusion therapy to individuals with acute or chronic conditions requiring administration of home infusion drugs;
- Ensures the safe and effective provision and administration of home infusion therapy on a 7-day-a-week, 24-hour-a-day basis; and
- Is accredited by an organization designated by the Secretary pursuant to section 1834(u)(5).
Both involve the administration of parenteral medications via various accesses and ports, with care provided by qualified professional nurses, as allowed by state regulations.
Pharmacy IRN services are provided in a variety of settings. Home infusion therapy services are provided in the client’s/patient’s home.
The most important difference is that Home Infusion Therapy Accreditation allows an organization to bill Medicare Part B and third-party payors with a physician’s order.
Separate clinical records must be kept for patients receiving IRN or home infusion therapy supplier (HITS) services.
Infusion pharmacies that provide infusion nursing services may want to consider achieving Home Infusion Therapy Accreditation if they employ or contract for nursing services and plan to bill Medicare Part B for infusion nursing services.
A qualified home infusion therapy supplier may subcontract with a pharmacy, physician, provider of services, or another supplier to meet Centers for Medicare & Medicaid Services (CMS) requirements. The home infusion therapy supplier is responsible for negotiating appropriate contract terms in order to assume responsibility only for services related to home infusion therapy.
The Centers for Medicare & Medicaid Services (CMS) provides a description of home infusion therapy for patients at home, an applicable section of the 21st Century Cures Act, a searchable directory of suppliers, payment policy, and other information at
https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/Home-Infusion-Therapy/Overview.html.
Hospice
There are no capitalization requirements for a hospice. A hospice may have its own capitalization budget for future growth, adding an inpatient unit, etc., but it is the hospice governing board’s decision on when to use it. However, some states may have a capitalization requirement through their licensure or Certificate of Need (CON).
The organization must have provided care to a minimum of five (5) patients (not required to be Medicare beneficiaries). At least three (3) of the required five (5) patients must be receiving care at the time of the Initial Medicare Certification Survey, unless care is provided in a medically underserved or rural area as determined by the Regional Office.
Yes. All hospice providers, regardless of the ability to bill, must be able to provide all four levels of care: routine, respite, continuous, and short-term inpatient.
The ACHC compliance date is the date at which you acknowledge that your organization was/is/will be in compliance with the ACHC Accreditation Standards. The compliance date does not apply to state and Medicare Conditions of Participation (CoPs) because agencies must always be in compliance with these requirements from the initiation of patient care.
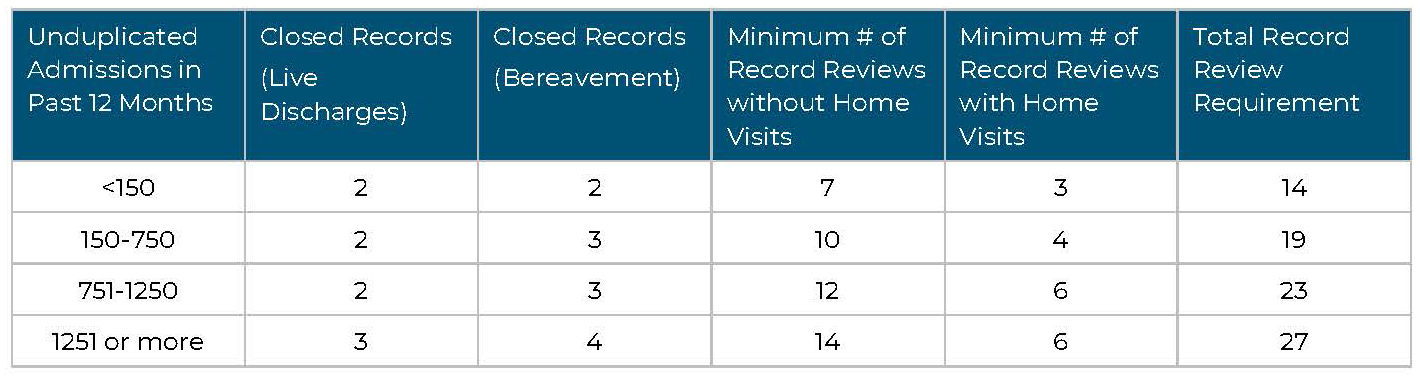
The number of medical records and home visits will be determined by your unduplicated admissions for the past 12 months or since the start of business, if a new agency. The chart below details the number of patient medical records and home visits that are required per unduplicated admissions.
Hospice Sample Record Review and Home Visit Requirements
No. The Surveyor will review patient records based on the care that has been provided up to the date of survey.
Yes. The Surveyor will choose a sampling of patient records and may conduct home visits of patients that are in different settings, such as a skilled nursing facility/nursing facility (SNF/NF), intermediate care facility, or facilities for individuals with intellectual disabilities (ICF/IID).
The Surveyor will choose a sampling of patient records and may conduct home visits of patients receiving different levels of care, such as routine, respite, general-inpatient, and continuous care.
The Surveyor will choose a sampling of active and closed records, representing different diagnoses, levels of care, and locations where care is being provided. The Surveyor will choose a sampling of patients with the following diagnoses:
- Dementia
- Stroke
- Circulatory/heart conditions
- Cancer
- Respiratory
- Chronic kidney disease
Yes. The Centers for Medicare & Medicaid Services (CMS) requires hospices to provide all core services — physician, nursing, social work, and counseling services (spiritual, bereavement, and dietary) — directly by hospice employees. Physician services are the exception and can be provided by employees, under arrangement, or by a combination of employees and under arrangement.
All noncore services — aide, physical therapy, occupational therapy, speech-language pathology, homemaker, volunteer, and medical supply services — can be provided by employees or under arrangement.
No. Neither CMS nor ACHC require hospice providers to have an EMR system. Medical record documentation can be a paper record.
No. A homebound status requirement does not apply to hospice patients.
No. For the survey to be valid, CMS requires all patient visits to be conducted in person and in the patient’s residence.
No. A provider is not allowed to charge Medicare beneficiaries privately for their care. Beneficiaries can opt to receive hospice care from other providers already certified by Medicare and use their Medicare hospice benefit to pay for services.
Office-Based Surgery
Surveys for OBS Accreditation are announced. Your Account Advisor as well as our scheduling team will collaborate with you to select a date that works best for your team.
A Preliminary Survey Assessment (PSA) is conducted on an organization that applies for ACHC Accreditation for the first time and has not begun providing care, treatment, or services. A Preliminary Survey Assessment is limited in scope, addressing a subset of administrative and organizational standards. Following this preliminary survey, an organization receives a Preliminary Accreditation status allowing it to serve patients for the purpose of seeking a full accreditation status within six (6) months. An organization within the U.S. or its territories may apply for preliminary accreditation if it has applied for a state license, occupies a properly equipped building, has developed policies meeting current Accreditation Requirements for Office-Based Surgery, and has appointed a chief executive, an administrator, or a clinical director.
Yes, ACHC provides resources and tools to help office-based surgery (OBS) organizations prepare for accreditation surveys. This includes a free self-assessment tool that allows new applicants to conduct their own mock surveys and set themselves up for success. In addition to tools, an office-based surgery will have access to our Clinical Review Specialists team for information and guidance.
Palliative Care
Palliative care services can be provided in a variety of settings, including a patient’s home, a clinic, a physician’s office, a long-term care facility, and an assisted living community.
Your organization must have cared for at least five palliative care patients and have three actively receiving care at the time of survey.
No. There currently is no full Medicare benefit for palliative care services. However, Medicare Part B, as well as some Medicaid programs and private insurance providers, may cover certain services related to palliative care, such as visits from physicians or advanced practice clinicians.
Some Medicare Advantage plans and some state Medicaid plans pay for palliative care services. The plans vary on what is covered. Organizations should investigate plans available in their service area.
The hospice Medicare benefit requires a physician to certify that a patient has a life expectancy of six (6) months or less and is forgoing curative treatment. Palliative care does not have such limitations.
Palliative care provides specialized services for patients with serious illnesses, regardless of age or prognosis. It can be provided along with curative treatment. Patient eligibility criteria are established by the program.
No. Hospice care is palliative care, and the intent is that once a patient is appropriate or ready for hospice care, the patient is referred to a hospice provider.
Yes. Palliative care patients may also have a skilled care need that must be managed by a home health provider.
Pharmacy/PCAB
THIRD-PARTY RECOGNITION – PCAB Accreditation meets compliance requirements for a growing number of payors and networks.
IMPROVED QUALITY AND SAFETY – In achieving PCAB Accreditation, pharmacies benefit from consistent practices that result in improved safety, efficiency, and quality of care.
RISK AVERSION – Adherence to PCAB standards helps pharmacies maintain compliance with all applicable USP guidelines.
MARKET ADVANTAGE – PCAB Accreditation allows pharmacies to distinguish themselves among their competitors by demonstrating a commitment to compliance with USP guidelines as well as industry best practices.
OPERATIONAL EFFICIENCIES – PCAB’s educational approach to accreditation enhances business operations, helps inform effective strategies, and improves patient outcomes through evidence-based best practices.
CONTINUITY OF SERVICE – PCAB facilitates a standardized level of service that includes sound procedures, documentation, and training to ensure consistent performance across the entire organization.
To have our Surveyors best evaluate your compounding processes, we ask that you have completed 30 to 90 days of compounding. Recordkeeping, ingredient selection, clean room certification, and personnel training and competencies should also be completed. If you are not currently compounding, please discuss your situation with your Account Advisor.
PCAB remains the gold standard in compounding accreditation, providing pharmacies with a comprehensive peer review of practices and corrective measures to achieve compliance with USP guidelines. Achieving PCAB Accreditation confirms your commitment to meeting the highest industry standards for quality and safety.
AIS inspections are a state-approved service for pharmacies that perform compounding, regardless of accreditation status. Inspection criteria are customized for each state. AIS inspections verify that state board of pharmacy requirements and USP compounding standards are met, including by nonresident compounding pharmacies.
IRX Accreditation is chosen by pharmacies that need a Medicare Part B billing number to receive reimbursement for infusion pumps and related supplies. Infusion Pharmacy services include IV drug mixture preparation, IV administration, therapy monitoring, client/patient counseling, and education. Accreditation confirms compliance with USP standards for sterile and non-sterile preparations.
PCAB Accreditation is appropriate for pharmacies in a variety of settings that want to demonstrate compliance with USP standards for sterile and/or non-sterile compounding.
PCAB Accreditation offers the most comprehensive compliance solution in the industry, with standards based on U.S. Pharmacopeia (USP) requirements. Achieving PCAB Accreditation demonstrates your commitment to meeting the highest industry standards for quality and safety.
PCAB reviews pharmacies that compound medications in retail, hospital, mail order, or closed-door settings.
Updates to USP compounding standards take effect November 1, 2023. Until that date, ACHC Surveyors are surveying to USP General Chapter <795> for non-sterile compounding, 2014 version, and USP General Chapter <797> for sterile compounding, 2008 version. Facilities implementing the 2023 updates before the November deadline will not be cited during their accreditation survey for following the revised standards.
Renal Dialysis
A dialysis facility seeking Initial Medicare Certification must have an approved CMS-855A Medicare Enrollment Application – Institutional Providers Form from the Centers for Medicare & Medicaid Services (CMS) and submit that approval letter to Accreditation Commission for Health Care (ACHC) before an accreditation survey can be conducted.
ACHC gained full deeming authority from CMS in April 2019, therefore the accreditation survey completed by ACHC takes the place of a CMS/State Agency survey. Once your survey is complete and any deficiencies cited have been corrected and approved by ACHC, we will submit to CMS the final regulatory packet recommending your approval. CMS will issue the official CMS Certification Number (CCN) and approval letter with the date recommended by ACHC.
Medical records will be reviewed for at least 10% of the total number of patients on census (minimum of four). Records should represent all modalities provided at the facility. During an initial survey, all medical records will be reviewed if the patient census is less than four.
The number of locations visited depends upon the number of skilled nursing/long-term care facilities in which the dialysis provider is performing dialysis treatments. If multiple locations are being served, a minimum of two locations will be visited, with the possibility of more based on the Surveyor’s observations.
Facilities seeking Initial Medicare Certification are not eligible for a medical director waiver. A waiver is permitted only for a dialysis facility that already has a CMS Certification Number (CCN) and is seeking a waiver for a temporary time frame due to extreme circumstances.
ACHC follows the same protocols as a CMS/State Agency survey: All deemed program surveys are unannounced.
Transitioning to ACHC is an easy process requiring just a few steps to set up your account. Once you have submitted your information, an Account Advisor will be assigned to you and will help guide you through the process. You will receive a letter of notification from ACHC that you can provide to your State Agency, informing the agency of your decision to use ACHC for your recertification/accreditation survey.
Telehealth Certification
Telehealth is the provision of care to patients with acute or chronic conditions using telehealth technology to allow monitoring in the home environment and other remote locations.
Use of telehealth technology creates disease management empowerment and independence, improved access to care, increased collaboration among healthcare providers, and improved patient outcomes.
Telehealth may include remote patient monitoring (RPM), biometrics, video, talk, or education. ACHC Standards for Telehealth Certification are based on the American Telemedicine Association’s Home Telehealth Clinical Guidelines.
Telehealth and telemedicine are two different types of online healthcare services. Telemedicine refers specifically to online doctor visits, while telehealth also includes health-related education services like diabetes management or nutrition courses and health-related training.
Certification is a vital tool to optimize and strengthen your organization. ACHC Certification is a focused review and evaluation of a defined program within a healthcare organization as measured against recognized standards for specialty care.
Certification, like accreditation, requires a survey — an independent, third-party review of your organization. ACHC uses an educational, collaborative survey approach that helps drive performance improvement, operating efficiencies, and risk management strategies.
By achieving certification, you demonstrate your commitment to high standards for quality, safety, and consistency.
Accreditation is independent third-party validation, through a comprehensive survey process, that a healthcare organization’s policies, processes, and care delivery meet recognized standards for quality and safety.
Certification is a focused review and evaluation of a defined program within a healthcare organization as measured against recognized standards for specialty care.
An ACHC award of Distinction is based on a focused review and evaluation of a defined specialty service offered by an ACHC-accredited healthcare organization as measured against recognized standards for specialty care.
All three categories focus on improving patient outcomes through effective processes that ensure the individual needs of patients are met with the highest-quality care and services. Each recognizes an organization’s commitment to continuous quality improvement, business efficiency, and safety.
Telehealth services have expanded dramatically during the COVID-19 pandemic and are expected to remain an important part of the healthcare continuum.
ACHC Telehealth Certification establishes national standards to promote best practices for delivering the highest-quality digital healthcare services. Certification confirms the quality of your services — strengthening trust in your organization and assuring patients they are receiving the best care.
As telehealth adoption increases, payors are likely to require third-party validation, like certification, to distinguish quality of care and validate service delivery. Our standards were developed with the future in mind, keeping you ready to meet possible payor requirements.
Telehealth Certification does not require accreditation from ACHC or another accreditor. It is available for any healthcare individual or organization that delivers health-related services via electronic information and telecommunication technologies.
The Distinction in Telehealth requires ACHC Accreditation. To apply for the Distinction in Telehealth, you must be accredited or seeking accreditation in one of seven ACHC programs: Ambulatory Care, Behavioral Health, Home Health, Hospice, Palliative Care, Private Duty, and Renal Dialysis.
If you are already accredited by ACHC in any of these programs or are seeking accreditation in any of them, it is recommended that you apply for the Distinction in Telehealth. Please contact your Account Advisor for more information.
If you are accredited by ACHC under a different program or are not accredited and do not want to be accredited, you should apply for ACHC Telehealth Certification.
Both Telehealth Certification and the Distinction in Telehealth focus on the provision of health care via telehealth technology to promote best practices for optimal care, privacy, cybersecurity, and safety.
Standards were developed in collaboration with leading industry associations and experts and are based on the American Telemedicine Association’s Home Telehealth Clinical Guidelines.
ACHC Telehealth Certification Standards are available to download from ACHC’s Customer Portal.
As a telehealth provider, delivering quality care and services is your highest priority. Achieving ACHC Telehealth Certification confirms your dedication to providing the best care and services.
Benefits include:
- Accountability
Confirm your commitment to quality, patient privacy and safety, cybersecurity, and consistency. - Credibility
Elevate your organization’s standing and gain industry credibility. - Minimized risk
Maximize value while minimizing risk. - Competitive advantage
Stand out among competitors. Be ready for possible payor requirements.
Your goals are our goals. ACHC understands the trust placed in your organization for delivering reliable, first-rate care and services.
Like you, we focus on improving patient outcomes. With your needs in mind, we use a more collaborative, educational approach that helps you continuously enhance the quality of your services and improve operational efficiencies.
ACHC’s cost-effective, streamlined process is designed to help you quickly and easily achieve certification. Our dedicated staff is ready to assist you before, during, and after your survey. Educational resources also are available to support your team and further your success.
At ACHC, we are more than an accreditor. We focus on empowering you to meet your strategic goals — and build a lasting partnership along the way.
Organizations are welcome to contact ACHC and explore their options for recognition of telehealth services. ACHC staff is available to discuss the best solutions for your organization.
Call (855) 937-2242 or email [email protected] to learn more.
ACHC staff can give you a quote personalized to meet your needs. Call (855) 937-2242 or email [email protected] to learn more.
The certification cycle is three years.
The total number of survey days is typically from one to two days but is determined by the total weekly visit volume.
A minimum of five client/patient charts will be reviewed. The total number of charts reviewed will be based on the organization’s total weekly visit volume.
The Surveyor will review the records or files for personnel delegated as responsible for telehealth services and additional personnel records for staff providing telehealth care/services.
Yes. A dependent survey result could be determined, depending on the number and type of deficiencies identified during the survey process.
 Accreditation Commission for Health Care, Inc.
Accreditation Commission for Health Care, Inc.